Three scientists. Three precious scientists saved the human race from complete demise. Three. Only three.
The USA needs to remember what values existed during some of the most astounding finds in science, literature, physics, medicine, chemistry and peace. The culture of a country matters. It creates the basis of which continued living on Earth exists.
Three scientists is the reason human beings are still living and breathing today on Earth. No one should forget that. When 97 percent of scientists on Earth state anthropogenic global warming is real, now and needs to end; everyone should be listening!
In 1971, H. S. Johnston, at the University of California (Berkeley), pointed out the potential danger of a large fleet of SSTs emitting considerable amounts of nitric oxide into the lower stratosphere, possibly accelerating natural ozone destruction. Only three years later,
F. S. Rowland and M. Molina (click here) showed that a widely used class of very inert chemicals known as chloro- fluorocarbons were transported to the stratosphere by convective air movements. There, they could absorb high-energy photons from sunlight and release free chlorine; Once released, the chlorine could destroy stratospheric ozone through a series of catalytic reactions....
(published in The Chapman & Hall Encyclopedia of Environmental Science, edited by David E. Alexander and Rhodes W. Fairbridge, pp pp.78-80, Kluwer Academic, Boston, MA, 1999.)
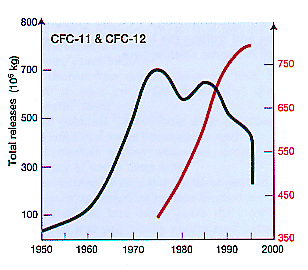
Chlorofluorocarbons (CFCs) are nontoxic, nonflammable chemicals containing atoms of carbon, chlorine, and fluorine. They are used in the manufacture of aerosol sprays, blowing agents for foams and packing materials, as solvents, and as refrigerants. CFCs are classified as halocarbons, a class of compounds that contain atoms of carbon and halogen atoms. Individual CFC molecules are labeled with a unique numbering system. For example, the CFC number of 11 indicates the number of atoms of carbon, hydrogen, fluorine, and chlorine (e.g. CCl3F as CFC-11). The best way to remember the system is the "rule of 90" or add 90 to the CFC number where the first digit is the number of carbon atoms (C), the second digit is the number of hydrogen atoms (H), and the third digit is number of the fluorine atoms (F). The total number of chlorine atoms (Cl) are calculated by the expression: Cl = 2(C+1) - H - F. In the example CFC-11 has one carbon, no hydrogen, one fluorine, and therefore 3 chlorine atoms....